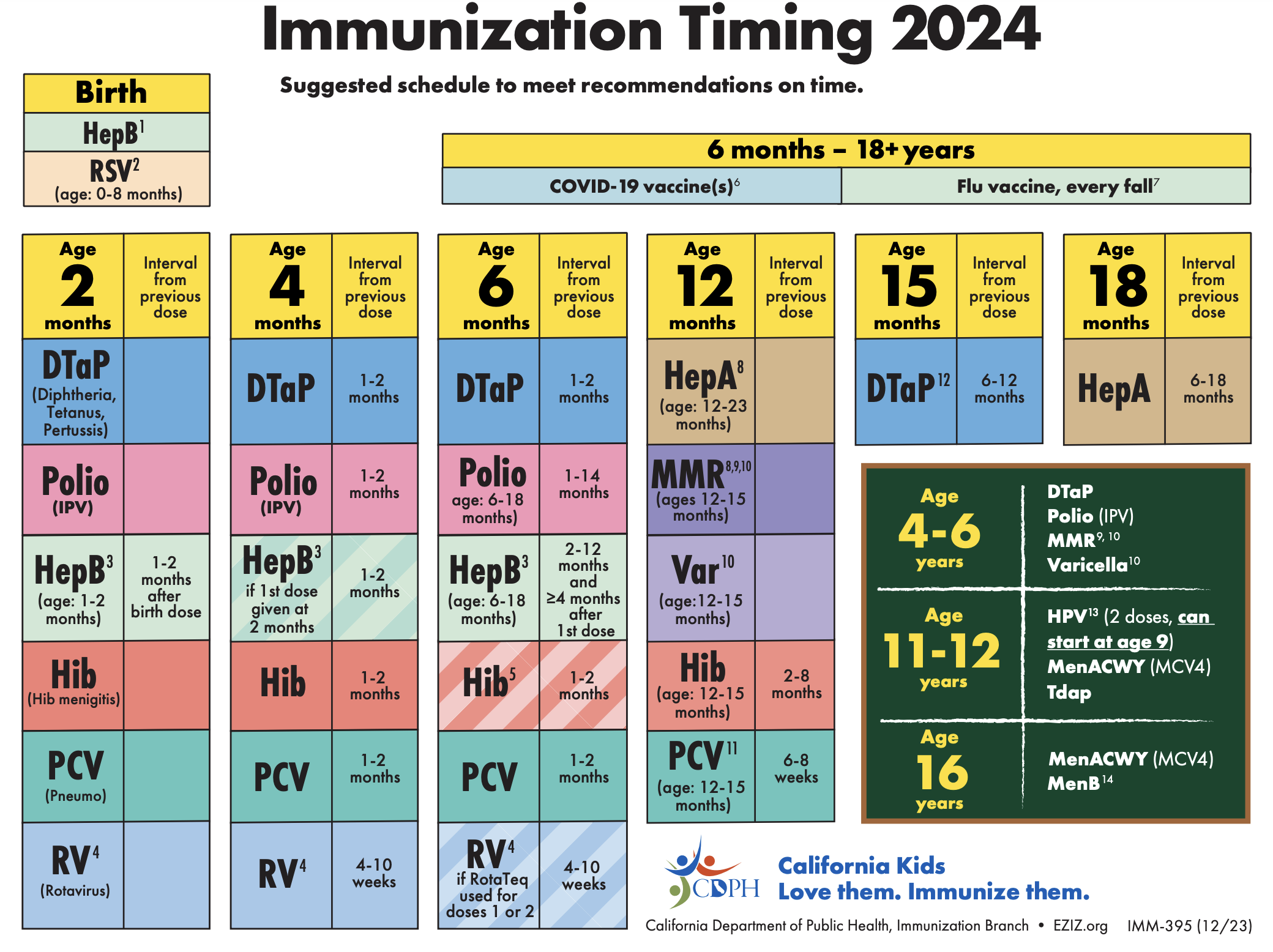
This is a suggested schedule. For alternatives and details, including additional recommendations for high-risk children, consult the Recommended Child and Adolescent Immunization Schedule for ages 18 years or younger, United States,
2024.
- Monovalent HepB vaccine is recommended within 24 hours of birth for stable infants weighing >2 kg. For others, see schedule.
- Infants <8 months entering their first RSV season should receive RSV immunization if birth parent’s prenatal RSV vaccination status is: unvaccinated, unknown, or vaccinated <14 days before birth. RSV immunization is also recommended for specified children ages 8-19 months who are at increased risk of severe RSV and entering their 2nd RSV season.
- A dose of HepB vaccine is not necessary at 4 months if doses are given at birth and 2 months but may be included as part of a combination vaccine. The final dose (3RD/4TH) should be given after age 24 wks. and at least 16 wks. after 1st dose.
- Administer first dose at age 6 wks-14 wks. (Max. age: 14 wks., 6 days). Max. age for final dose in the series: 8 months, 0 days. If any dose of RV5 is given or product is unknown, a total of three RV doses are needed.
- This 6 month Hib dose is not indicated if PedvaxHIB® is used exclusively for the 2 and 4 month infant doses.
- See CDC guidelines for doses and intervals for healthy or immunocompromised children.
- Two doses given at least 4 weeks apart are recommended for ages 6 months–8 years who are getting flu vaccine for the first time.
- Refer to CDC guidelines for vaccinating children 6-11 months prior to international travel.
- Min. interval between 1ST and 2ND dose is 4 wks. Two MMR doses should still be given on or after 12 months of age.
- Minimum intervals: Ages 1-12 year: 3 months. Ages 13 years and older: 4 weeks. MMRV may be used when both MMR and Varicella vaccines are indicated. For the 1st dose at 12-15 months, MMR and varicella vaccines should typically be given unless the parent or caregiver prefers MMRV.
- Final dose of PCV series should be given at ≥12 months of age or after.
- The 4th dose of DTaP may be administered as early as 12 months, provided at least 6 months have elapsed since the 3RD DTaP dose.
- HPV vaccine should be given on a 0, 6-12 month schedule for 9-14 year olds (min. interval is 5 months). If patient immunocompromised or initiates series at 15 years or older, use a 3 dose schedule (0, 1-2, 6 months).
- A MenB vaccine series may be given to all persons 16 through 23 years of age. See MMWR for details. Pentavalent MenABCWY vaccine may be used when both MenACWY and MenB are indicated at the same visit. This publication was supported by Grant Number H23/CCH922507 from the Centers for Disease Control and Prevention (CDC). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of CDC.
This publication was supported by Grant Number H23/CCH922507 from the Centers for Disease Control and Prevention (CDC). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of CDC.